
Danone said today it welcomed the news Fonterra’s products had been given the all-clear on botulism contamination.
Danone, which sources ingredients from New Zealand-based Fonterra and had to recall products as a result of the scare, added that it was “currently reviewing its recourse and compensation options”.
Read this
Botulism or not? Making sense of the Fonterra tests
The botulism whey scare in New Zealand has turned out to be a false alarm. How did the experts get it so wrong?
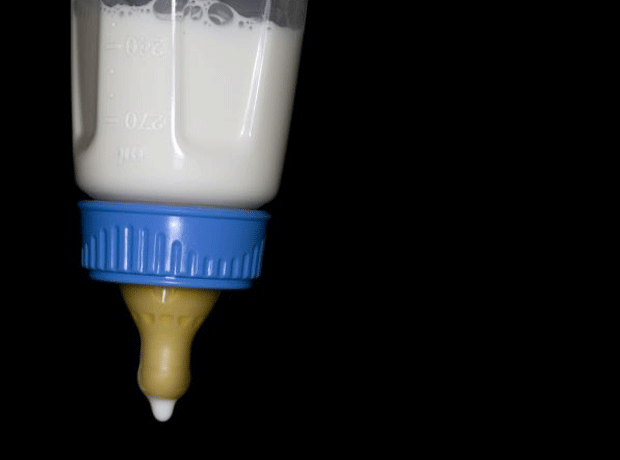
Tests by New Zealand authorities revealed this week that the botulism warning over Fonterra’ whey products was a false alarm. Earlier tests by a separate New Zealand lab had suggested whey protein concentrate – used to make baby formula - had been contaminated with Clostridium botulinum via a dirty pipe.
The scare led to a large-scale recall of products from key markets such as China, and import restrictions being imposed on Fonterra.
Danone recalled selected infant formula products from sale in eight markets in the Asia-Pacific region as a precautionary measure. It said these recalls had “a significant impact” on sales in its Baby Nutrition division.
“The division’s third-quarter sales will be down, but despite this, our group is on track to deliver organic growth of around 5% this quarter. We are deploying action plans to restore sales in affected markets. Their success will enable Danone to meet its growth and margin targets for 2013,” said Danone’s chief financial officer Pierre-André Térisse.
Danone said it had also incurred costs associated with recall procedures and “efforts to boost sales”.
















No comments yet